1. 不銹鋼(gang)化(hua)學鍍銅的(de)應用
不銹(xiu)鋼化學鍍銅應用于電子工(gong)業、計算(suan)機(ji)工(gong)業及航空(kong)工(gong)業中(zhong)電子元件的(de)高效電磁(ci)干擾的(de)屏蔽。
2. 不銹(xiu)鋼基體(ti)上(shang)化學鍍銅存(cun)在(zai)的問題(ti)
在線亞洲日產一區二區:不銹鋼基體上化學鍍銅易造成鍍層鼓泡,這不僅影響了鍍層與基體的結合力,而且直接影響到外觀質量。為此,將鍍前酸處理過的不銹鋼放在烘箱中加熱,以除去酸洗時滲入到基體的氫,采用此方法解決了鍍層起泡問題,得到所需要的化學鍍銅層。
3. 不銹(xiu)鋼化學鍍(du)銅工藝(yi)流程
NiCr不銹(xiu)(xiu)鋼(經(jing)過600℃真空熱處(chu)理(li))→化(hua)學(xue)除(chu)油[氫(qing)氧化(hua)鈉(NaOH)10%(質量分數(shu))]→水洗(xi)(xi)(xi)→熱水洗(xi)(xi)(xi)→除(chu)銹(xiu)(xiu)(鹽酸1:1溶液,溫度(du)80~100℃,時間5min)→水洗(xi)(xi)(xi)→干燥(zao)→除(chu)氫(qing)(在烘箱中溫度(du)200℃,時間2h)→酸處(chu)理(li)[稀硫(liu)酸5%(質量分數(shu)),時間1~5min]→水洗(xi)(xi)(xi)→去離子水洗(xi)(xi)(xi)→化(hua)學(xue)鍍銅→水洗(xi)(xi)(xi)→抗銅變色處(chu)理(li)(苯并三氮唑(zuo)1g/L,溫度(du)65℃,時間2min)→純水洗(xi)(xi)(xi)→熱純水洗(xi)(xi)(xi)→干燥(zao)。
4. 化學鍍銅溶(rong)液(ye)成分及工藝條(tiao)件見表(biao)4-39
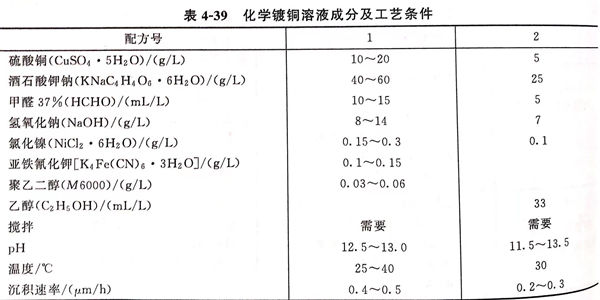
5. 化學(xue)鍍銅(tong)溶液的(de)配制(zhi)
先將(jiang)硫酸(suan)銅(tong)和酒(jiu)石(shi)酸(suan)鉀(jia)鈉分別用(yong)純(chun)水(shui)(shui)溶(rong)(rong)(rong)解(jie)(jie)(jie),然后(hou)將(jiang)硫酸(suan)銅(tong)溶(rong)(rong)(rong)液在攪(jiao)拌(ban)下(xia)加(jia)入酒(jiu)石(shi)酸(suan)鉀(jia)鈉溶(rong)(rong)(rong)液中(zhong),銅(tong)離子被酒(jiu)石(shi)酸(suan)離子絡(luo)(luo)合(he)(he)成藍(lan)色絡(luo)(luo)合(he)(he)物。再將(jiang)氯化(hua)(hua)鎳用(yong)少量(liang)水(shui)(shui)溶(rong)(rong)(rong)解(jie)(jie)(jie)后(hou)攪(jiao)拌(ban)加(jia)入,再加(jia)入甲醛溶(rong)(rong)(rong)液,攪(jiao)拌(ban)均(jun)勻。將(jiang)氫(qing)氧(yang)化(hua)(hua)鈉用(yong)純(chun)水(shui)(shui)溶(rong)(rong)(rong)解(jie)(jie)(jie)成200g/L 的濃溶(rong)(rong)(rong)液待用(yong)。在開始化(hua)(hua)學鍍(du)銅(tong)前,逐步在攪(jiao)拌(ban)下(xia)加(jia)入藍(lan)色絡(luo)(luo)合(he)(he)液中(zhong),使溶(rong)(rong)(rong)液pH達到12 左(zuo)右(用(yong)9~13精密pH試(shi)紙測量(liang)),最(zui)(zui)后(hou)將(jiang)穩定(ding)劑亞鐵氯化(hua)(hua)鉀(jia)、聚乙(yi)二醇用(yong)少量(liang)水(shui)(shui)溶(rong)(rong)(rong)解(jie)(jie)(jie)后(hou)攪(jiao)拌(ban)加(jia)入,乙(yi)醇可直(zhi)接加(jia)入,最(zui)(zui)后(hou)用(yong)純(chun)水(shui)(shui)加(jia)入至溶(rong)(rong)(rong)液的規定(ding)體積(ji),攪(jiao)拌(ban)均(jun)勻后(hou)放(fang)入不(bu)銹鋼件即可開始化(hua)(hua)學鍍(du)銅(tong)。
6. 操作要點(dian)
①. 裝載量
按照(zhao)每(mei)升鍍液裝載2d㎡計算。
②. 除氫和攪拌
不銹鋼對氫(qing)(qing)滲(shen)(shen)(shen)很敏感,工(gong)(gong)(gong)件在(zai)酸(suan)(suan)洗(xi)(xi)過(guo)程中(zhong)氫(qing)(qing)會(hui)滲(shen)(shen)(shen)人到基(ji)體(ti)(ti)中(zhong),如果(guo)不除氫(qing)(qing),化學鍍(du)(du)(du)(du)銅鍍(du)(du)(du)(du)層(ceng)(ceng)(ceng)致密小孔覆蓋在(zai)不銹鋼表(biao)(biao)(biao)面(mian)后,氫(qing)(qing)氣(qi)(qi)無法逸出,造成(cheng)很大的(de)(de)應(ying)(ying)力(li),使(shi)(shi)鍍(du)(du)(du)(du)層(ceng)(ceng)(ceng)起泡(pao)(pao),加上化學鍍(du)(du)(du)(du)銅本身伴隨著(zhu)析氫(qing)(qing)過(guo)程,氫(qing)(qing)氣(qi)(qi)會(hui)殘留在(zai)基(ji)體(ti)(ti)與(yu)鍍(du)(du)(du)(du)層(ceng)(ceng)(ceng)金屬的(de)(de)晶格中(zhong),增大內應(ying)(ying)力(li),嚴重地減弱(ruo)基(ji)體(ti)(ti)與(yu)鍍(du)(du)(du)(du)層(ceng)(ceng)(ceng)的(de)(de)結合(he)(he)強(qiang)度(du)(du)。為此,從兩方面(mian)著(zhu)手解(jie)(jie)決鍍(du)(du)(du)(du)層(ceng)(ceng)(ceng)起泡(pao)(pao)問(wen)題。其(qi)一是把經(jing)過(guo)去油(you)、酸(suan)(suan)洗(xi)(xi)后的(de)(de)工(gong)(gong)(gong)件在(zai)化學鍍(du)(du)(du)(du)銅前進行熱(re)處(chu)理(li),除去滲(shen)(shen)(shen)入(ru)到基(ji)體(ti)(ti)中(zhong)的(de)(de)氫(qing)(qing),熱(re)處(chu)理(li)溫(wen)度(du)(du)和(he)時(shi)(shi)(shi)間條件經(jing)實(shi)驗(yan)確定(ding)為180~200℃,2小時(shi)(shi)(shi),鍍(du)(du)(du)(du)層(ceng)(ceng)(ceng)無鼓(gu)泡(pao)(pao),鍍(du)(du)(du)(du)層(ceng)(ceng)(ceng)結合(he)(he)力(li)合(he)(he)格。溫(wen)度(du)(du)過(guo)低或(huo)時(shi)(shi)(shi)間過(guo)短(duan)仍有輕微(wei)鼓(gu)泡(pao)(pao),溫(wen)度(du)(du)過(guo)高或(huo)時(shi)(shi)(shi)間過(guo)長都容(rong)易使(shi)(shi)表(biao)(biao)(biao)面(mian)再次生成(cheng)不易去除的(de)(de)氧化皮,又需要較(jiao)長時(shi)(shi)(shi)間的(de)(de)強(qiang)酸(suan)(suan)處(chu)理(li),酸(suan)(suan)洗(xi)(xi)時(shi)(shi)(shi)氫(qing)(qing)會(hui)再次滲(shen)(shen)(shen)入(ru)基(ji)體(ti)(ti)。在(zai)所選定(ding)的(de)(de)溫(wen)度(du)(du)和(he)時(shi)(shi)(shi)間下雖表(biao)(biao)(biao)面(mian)會(hui)有新的(de)(de)氧化膜(mo)生成(cheng),但(dan)使(shi)(shi)用稀硫酸(suan)(suan)短(duan)時(shi)(shi)(shi)間酸(suan)(suan)洗(xi)(xi)即可(ke),以免再次滲(shen)(shen)(shen)氫(qing)(qing)。其(qi)二(er)是在(zai)化學鍍(du)(du)(du)(du)銅過(guo)程中(zhong),采用某(mou)種(zhong)攪拌(ban)(空氣(qi)(qi)攪拌(ban)或(huo)機械(xie)攪拌(ban)),有利于銅離(li)子(zi)向工(gong)(gong)(gong)件表(biao)(biao)(biao)面(mian)擴散,防止和(he)減少副反應(ying)(ying)產物(wu)(wu)銅粉(即Cu2O)的(de)(de)生成(cheng),而且有利于反應(ying)(ying)產物(wu)(wu)氫(qing)(qing)氣(qi)(qi)脫離(li)工(gong)(gong)(gong)件表(biao)(biao)(biao)面(mian)。通過(guo)上述兩種(zhong)方法有效(xiao)地解(jie)(jie)決了鍍(du)(du)(du)(du)層(ceng)(ceng)(ceng)鼓(gu)泡(pao)(pao)問(wen)題,提高了鍍(du)(du)(du)(du)層(ceng)(ceng)(ceng)與(yu)基(ji)體(ti)(ti)的(de)(de)結合(he)(he)強(qiang)度(du)(du)。
③. 催化活性劑-鎳(nie)離子
在化(hua)學鍍(du)銅溶(rong)液中(zhong)加入少量(liang)(liang)鎳(nie)離子后,鍍(du)層性質(zhi)得到改善,在鍍(du)銅層中(zhong)含有微量(liang)(liang)的(de)鎳(nie),形成Cu89Ni11金(jin)屬化(hua)合物,它具(ju)有最佳(jia)的(de)催化(hua)活性,提高鍍(du)層的(de)催化(hua)活性。
④. 穩定劑(ji)的(de)控制
在(zai)化學鍍(du)(du)(du)(du)銅(tong)(tong)過程中(zhong),甲醛能將二價銅(tong)(tong)離子還(huan)原為金屬銅(tong)(tong)鍍(du)(du)(du)(du)層,還(huan)存在(zai)有副反(fan)(fan)(fan)應(ying),即不完全反(fan)(fan)(fan)應(ying)生成(cheng)暗紅色的(de)(de)(de)氧化亞銅(tong)(tong)(Cu2O),它形成(cheng)微(wei)粒(li)懸浮在(zai)鍍(du)(du)(du)(du)液(ye)(ye)中(zhong),呈膠體狀(zhuang)態,極(ji)難(nan)用過濾除去,若(ruo)與銅(tong)(tong)共沉積,使(shi)銅(tong)(tong)鍍(du)(du)(du)(du)層疏松粗糙,與基體結合(he)力極(ji)差。氧化亞銅(tong)(tong)被(bei)甲醛還(huan)原成(cheng)金屬微(wei)粒(li),又成(cheng)為自催化中(zhong)心,使(shi)鍍(du)(du)(du)(du)液(ye)(ye)自發分解,消耗了鍍(du)(du)(du)(du)液(ye)(ye)中(zhong)的(de)(de)(de)有效成(cheng)分。為了抑制(zhi)副反(fan)(fan)(fan)應(ying)的(de)(de)(de)發生,加入穩定(ding)劑,以提高鍍(du)(du)(du)(du)液(ye)(ye)的(de)(de)(de)穩定(ding)性(xing)(xing)。但是(shi),過量(liang)的(de)(de)(de)穩定(ding)劑的(de)(de)(de)加人,又成(cheng)了化學鍍(du)(du)(du)(du)銅(tong)(tong)反(fan)(fan)(fan)應(ying)的(de)(de)(de)催化毒性(xing)(xing)劑,顯著降低化學鍍(du)(du)(du)(du)的(de)(de)(de)速率(lv),甚至停鍍(du)(du)(du)(du),故選用穩定(ding)劑,并控制(zhi)其很(hen)低的(de)(de)(de)適宜含量(liang),對提高鍍(du)(du)(du)(du)液(ye)(ye)穩定(ding)性(xing)(xing)有效。
⑤. 防銅層變色(se)處理
對銅層(ceng)(ceng)進(jin)行防(fang)變(bian)色處理(li),在鍍銅層(ceng)(ceng)表面形成一層(ceng)(ceng)穩定的(de)絡合膜(mo),隔絕外界浸蝕性物質對鍍銅層(ceng)(ceng)的(de)作用(yong),使鍍銅層(ceng)(ceng)保持(chi)本色一定的(de)時間。苯并(bing)三氮唑要先用(yong)乙醇溶解好(hao),然后(hou)加入熱蒸餾(liu)水(shui)中(zhong)。防(fang)變(bian)色處理(li)的(de)溫度不低于65℃,時間不少于2min,否則防(fang)變(bian)色達不到效(xiao)果。
7. 鍍層(ceng)結合(he)強度檢測-劃痕實驗(yan)法
在鍍(du)層表面(mian)用(yong)刀片劃出(chu)1mm間(jian)距的直行(xing)線和90°交錯(cuo)的橫(heng)行(xing)線形成小方格。觀察劃痕交錯(cuo)處鍍(du)層有無起層,進一(yi)步用(yong)黏(nian)性(xing)高的膠帶貼于劃痕表面(mian),再撕下膠帶,以銅(tong)層不脫落為合格。
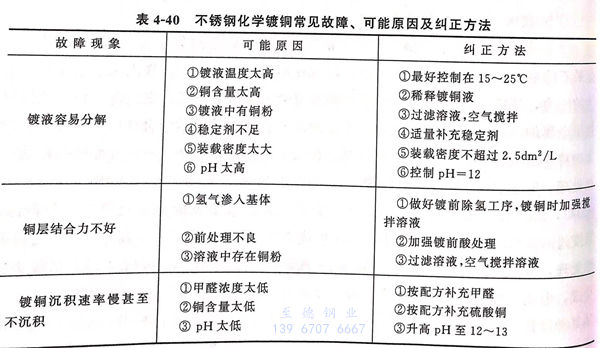
8. 不銹鋼(gang)化學鍍銅(tong)常見故障(zhang)、可能原(yuan)因及糾(jiu)正方法見表4-40.