從圖5.22中可以看出,不同表面粗糙度的試樣的極化曲線都具有活性溶解-鈍化轉變的特征,并且都具有三個自腐蝕電位。當電位低于Ecorr-1時,三條曲線的腐蝕電流密度相差很小。因此為了簡化討論可以認為具有不同表面粗糙的試樣的陰極反應(H+的還原反應)是相同的,不隨表面粗糙度的變化而變化。金屬的陽極溶解電流隨著表面粗糙度的增加而增加,這種現象可以用金屬的表面功函數(EWF)來解釋。EWF是指材料的自由電子脫離材料表面的最低能量。金屬的暴露面積越大材料失去電子被氧化的概率就越高,且EWF隨著材料的表面粗糙度的增加而降低,表面粗糙度大的試樣具有較低的EWF和較大的有效暴露面積,因此越容易失去電子,從而加速材料的腐蝕過程。
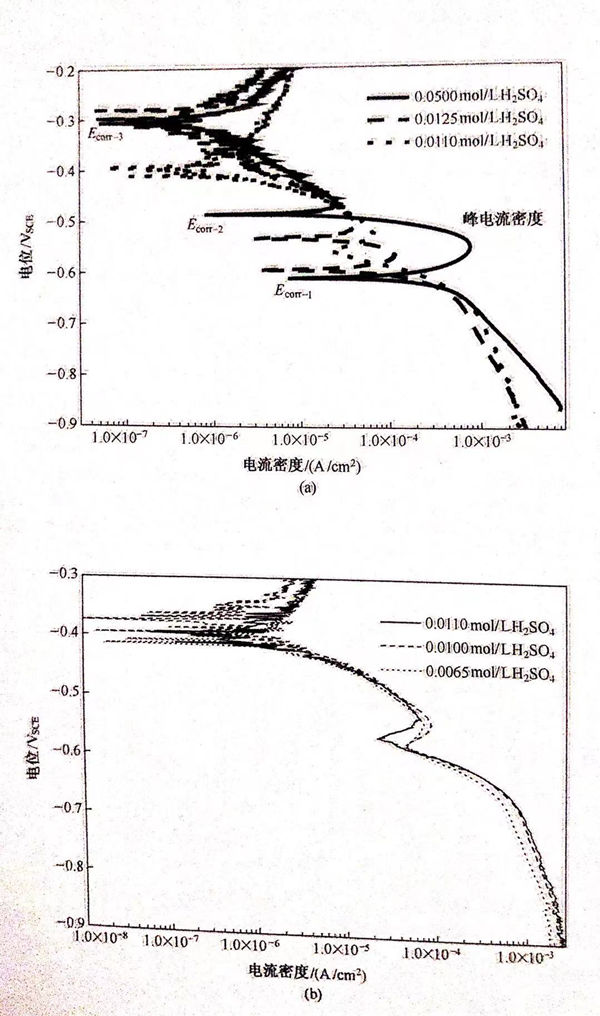
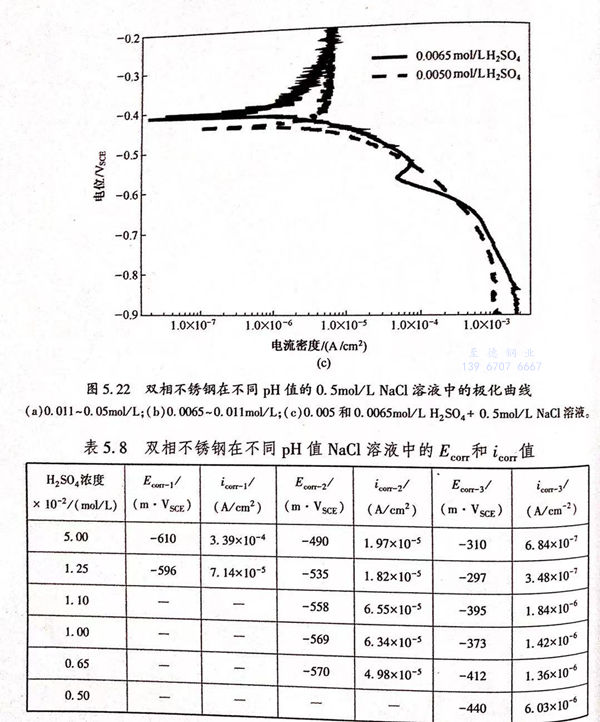
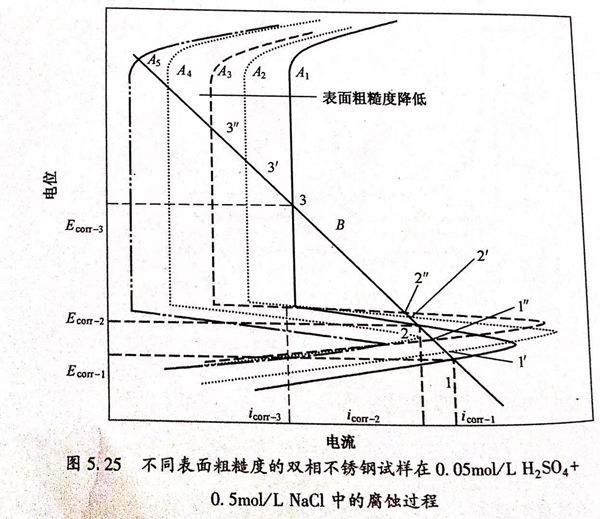
圖5.25的理論極化曲線可以用來解釋不同表面粗糙度的雙相(xiang)不銹(xiu)鋼的腐蝕過程。圖5.25中A1、A2、A3分別代表理論陽極極化曲線,B代表理論陰極極化曲線,陰、陽極極化曲線的交點個數即代表了自腐蝕電位的個數。假設B和A1分別代表240號砂紙打磨后的試樣的理論陰、陽極極化曲線,因此測量的極化曲線就是二者的代數疊加,從圖5.25中可以看出A1和B有三個交點(1、2、3),即表示有三個自腐蝕電位。當表面粗糙度降低(800號和拋光試樣)時,陽極極化曲線變為A2和A3,從圖5.25中可以看到仍然出現了三個交點(1'、2'、3')和(1”、2"、3”),即有三個自腐蝕電位。不同表面粗糙度的試樣的自腐蝕電位有如下關系:Ecorr-1<Ecorr1'<E corr-1",E corr-2<E corr-2,<E corr-2",E corr-3<Ecor-3/<Ecorr-3”。如果陽極極化曲線轉變為A4和A5,自腐蝕電位轉變為兩個或者一個,但是這種情況在本實驗條件下沒有出現。
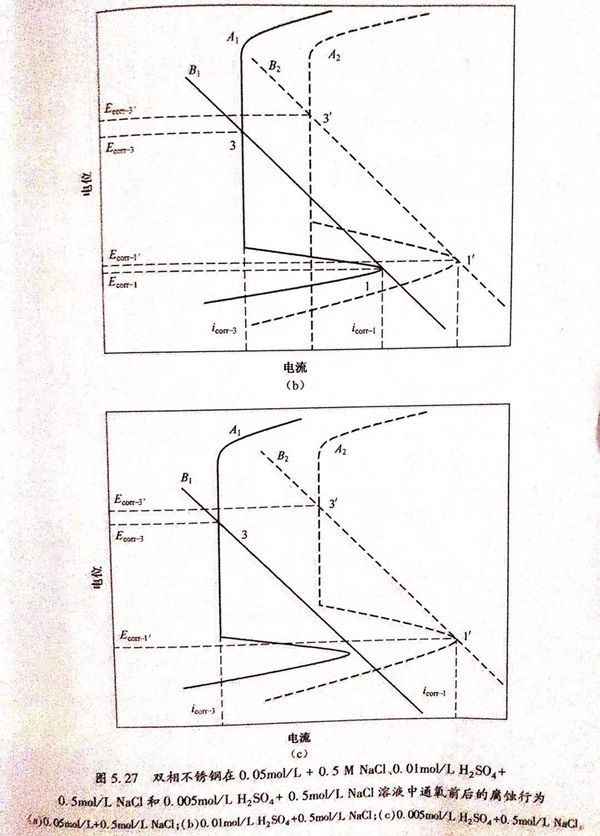
從圖5.22中可以看出,溶液的pH值可以顯著改變雙相不銹鋼在酸性NaCl溶液中的腐蝕行為,從而使自腐蝕電位的個數隨著H*濃度的降低而減少。在酸性NaCI溶液中,主要的陰極反應是H*的還原反應。可以用圖5.26中的理論極化曲線來解釋pH值改變對自腐蝕電位個數的影響。圖5.26中曲線A1、A2和A3分別代表雙相不銹鋼在不同pH值的溶液中的理論陽極極化曲線,B1、B2和B3代表陰極H+還原反應的理論極化曲線。如果腐蝕過程是由陰極曲線B1和陽極曲線A1耦合而成,那么在金屬的活性溶解區、溶解-鈍化過渡區和完全鈍化區內陰、陽極極化曲線就會有三個交點(1、2、3),也就會出現三個自腐蝕電位。這對應于圖5.22(a)中H2SO4濃度大于0.011mol/L的條件下的情況。降低溶液的pH值,就會影響陰、陽極反應的過程,從而改變極化曲線的位置。當陰、陽極極化曲線由B2和A2耦合而成,則只有兩個交點,也就是只有兩個自腐蝕電位存在。這對應于圖5.22(b)中H2SO4濃度介于0.0065~0.011mol/L時的條件下所得到的結果。進一步降低溶液的pH值,使硫酸濃度降為0.005mol/L以下,陰、陽極極化曲線由B3和A3耦合而成,則只有一個自腐蝕電位存在。若進一步降低溶液的pH值,自腐蝕電位的個數將不再發生改變,保持一個。
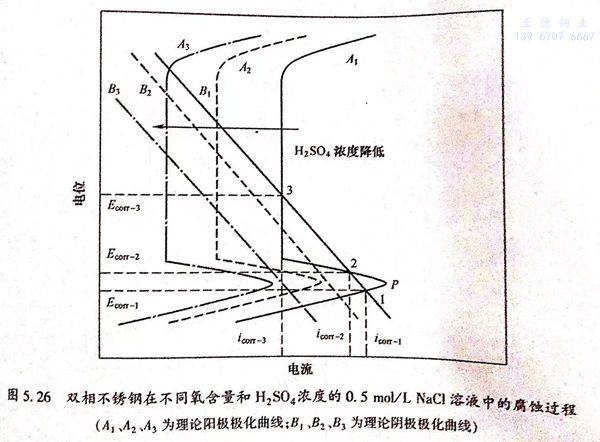
圖5.26 雙相不銹鋼在不同氧含量和H2SO4濃度的0.5mol/L NaCl溶液中的腐蝕過程(A1、A2、A3為理論陽極極化曲線;B1、B2、B3為理論陰極極化曲線)在有氧存在的條件下,溶液中的陰極反應除了H+的還原反應之外,還有氧的還原反應:
O2+4H++4e- = 2H2O
因此,在(zai)(zai)有氧(yang)(yang)存在(zai)(zai)條件下的(de)陰(yin)極(ji)反(fan)(fan)(fan)應的(de)電(dian)流(liu)密(mi)(mi)度(du)是氫還原(yuan)反(fan)(fan)(fan)應和(he)氧(yang)(yang)還原(yuan)反(fan)(fan)(fan)應的(de)電(dian)流(liu)密(mi)(mi)度(du)之和(he)。從(cong)圖(tu)5.24中可(ke)以看出(chu),溶(rong)液中的(de)氧(yang)(yang)不但能影(ying)(ying)響陰(yin)極(ji)反(fan)(fan)(fan)應過程(cheng),還能影(ying)(ying)響陽極(ji)反(fan)(fan)(fan)應過程(cheng),造成自腐(fu)蝕(shi)電(dian)位(wei)正移,腐(fu)蝕(shi)電(dian)流(liu)密(mi)(mi)度(du)增加(jia)。可(ke)能是由(you)于(yu)溶(rong)液中的(de)氧(yang)(yang)含量增加(jia),獲得自由(you)電(dian)子(zi)的(de)概率(lv)增加(jia);或(huo)者提(ti)高(gao)氧(yang)(yang)在(zai)(zai)金(jin)屬表(biao)面(mian)或(huo)者鈍化膜表(biao)面(mian)的(de)還原(yuan)速度(du)。
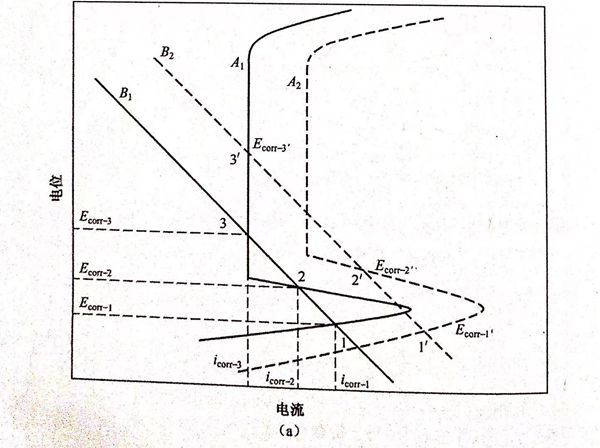
溶(rong)液中(zhong)的(de)(de)氧(yang)(yang)(yang)含量對雙(shuang)相不(bu)(bu)(bu)銹鋼在(zai)(zai)(zai)酸性NaCl溶(rong)液中(zhong)的(de)(de)腐蝕(shi)行(xing)(xing)為(wei)的(de)(de)影響(xiang)(xiang)(xiang)可(ke)以(yi)用圖5.27來(lai)解(jie)釋(shi)。氧(yang)(yang)(yang)對雙(shuang)相不(bu)(bu)(bu)銹鋼腐蝕(shi)行(xing)(xing)為(wei)的(de)(de)影響(xiang)(xiang)(xiang)與pH的(de)(de)影響(xiang)(xiang)(xiang)效果(guo)基本(ben)一致。圖5.27(a)可(ke)用來(lai)解(jie)釋(shi)圖5.23(a)的(de)(de)實驗(yan)現(xian)象。當溶(rong)液中(zhong)不(bu)(bu)(bu)含氧(yang)(yang)(yang)(氮氣飽(bao)和(he)(he))時(shi),B1和(he)(he)A1分別(bie)(bie)(bie)代表理(li)論的(de)(de)陰(yin)(yin)(yin)、陽(yang)(yang)(yang)極(ji)(ji)極(ji)(ji)化(hua)(hua)曲(qu)線(xian)(xian)。從圖5.27中(zhong)可(ke)以(yi)看(kan)出(chu)曲(qu)線(xian)(xian)有(you)(you)(you)三個(ge)(ge)(ge)(ge)交點(dian),即(ji)存(cun)(cun)在(zai)(zai)(zai)三個(ge)(ge)(ge)(ge)自(zi)腐蝕(shi)電(dian)位(wei)(wei) ECOrr-1、Ecorr-2和(he)(he)Ecorr-3相對應(ying)的(de)(de)腐蝕(shi)電(dian)流(liu)密(mi)度(du)為(wei)icorr-1、icorr-2和(he)(he)icorr-3.當有(you)(you)(you)氧(yang)(yang)(yang)存(cun)(cun)在(zai)(zai)(zai)(空氣飽(bao)和(he)(he)或氧(yang)(yang)(yang)飽(bao)和(he)(he))時(shi),陰(yin)(yin)(yin)、陽(yang)(yang)(yang)極(ji)(ji)極(ji)(ji)化(hua)(hua)曲(qu)線(xian)(xian)分別(bie)(bie)(bie)變為(wei)B2和(he)(he)A2,仍然有(you)(you)(you)三個(ge)(ge)(ge)(ge)自(zi)腐蝕(shi)電(dian)位(wei)(wei)Ecorr-1'、Ecorr-2'和(he)(he)Ecorr-3',并且 Ecorr-1'>E corr-1、Ecorr-21>Ecorr-2、Ecorr-3'>Ecorr-3。圖5.23(b)中(zhong)在(zai)(zai)(zai)通(tong)(tong)(tong)氧(yang)(yang)(yang)前后(hou)都只(zhi)有(you)(you)(you)兩(liang)個(ge)(ge)(ge)(ge)自(zi)腐蝕(shi)電(dian)位(wei)(wei),這一現(xian)象可(ke)以(yi)用圖5.27(b)來(lai)解(jie)釋(shi)。當溶(rong)液中(zhong)通(tong)(tong)(tong)入氧(yang)(yang)(yang)前,B1和(he)(he)A1分別(bie)(bie)(bie)代表理(li)論的(de)(de)陰(yin)(yin)(yin)、陽(yang)(yang)(yang)極(ji)(ji)極(ji)(ji)化(hua)(hua)曲(qu)線(xian)(xian),從圖5.27中(zhong)可(ke)以(yi)看(kan)出(chu)曲(qu)線(xian)(xian)有(you)(you)(you)兩(liang)個(ge)(ge)(ge)(ge)交點(dian),即(ji)存(cun)(cun)在(zai)(zai)(zai)兩(liang)個(ge)(ge)(ge)(ge)自(zi)腐蝕(shi)電(dian)位(wei)(wei)Ecorr-1和(he)(he)Ecorr-3,相對應(ying)的(de)(de)腐蝕(shi)電(dian)流(liu)密(mi)度(du)為(wei)icorr-1和(he)(he)icorr-3。當有(you)(you)(you)氧(yang)(yang)(yang)飽(bao)和(he)(he)時(shi),陰(yin)(yin)(yin)、陽(yang)(yang)(yang)極(ji)(ji)極(ji)(ji)化(hua)(hua)曲(qu)線(xian)(xian)分別(bie)(bie)(bie)變為(wei)B2和(he)(he)A2,仍然有(you)(you)(you)兩(liang)個(ge)(ge)(ge)(ge)自(zi)腐蝕(shi)電(dian)位(wei)(wei) Ecorr-1'和(he)(he)Ecorr-3'。通(tong)(tong)(tong)氧(yang)(yang)(yang)前后(hou)自(zi)腐蝕(shi)電(dian)位(wei)(wei)的(de)(de)關系為(wei)Ecorr-1'>Ecorr-1,Ecorr-3'>Ecorr-3.當硫酸濃度(du)為(wei)0.005mol/L時(shi),在(zai)(zai)(zai)極(ji)(ji)化(hua)(hua)曲(qu)線(xian)(xian)只(zhi)有(you)(you)(you)一個(ge)(ge)(ge)(ge)自(zi)腐蝕(shi)電(dian)位(wei)(wei)存(cun)(cun)在(zai)(zai)(zai),但溶(rong)液經過氧(yang)(yang)(yang)飽(bao)和(he)(he)后(hou),自(zi)腐蝕(shi)電(dian)位(wei)(wei)個(ge)(ge)(ge)(ge)數由(you)一個(ge)(ge)(ge)(ge)增加為(wei)兩(liang)個(ge)(ge)(ge)(ge),這一現(xian)象可(ke)以(yi)用圖5.27(c)來(lai)解(jie)釋(shi)。空氣飽(bao)和(he)(he)溶(rong)液中(zhong)的(de)(de)理(li)論陰(yin)(yin)(yin)、陽(yang)(yang)(yang)極(ji)(ji)極(ji)(ji)化(hua)(hua)曲(qu)線(xian)(xian)分別(bie)(bie)(bie)為(wei)B1和(he)(he)A1,從圖5.27中(zhong)可(ke)以(yi)看(kan)出(chu)只(zhi)有(you)(you)(you)一個(ge)(ge)(ge)(ge)交點(dian),即(ji)只(zhi)存(cun)(cun)在(zai)(zai)(zai)一個(ge)(ge)(ge)(ge)自(zi)腐蝕(shi)電(dian)位(wei)(wei);當氧(yang)(yang)(yang)氣飽(bao)和(he)(he)時(shi),由(you)于氧(yang)(yang)(yang)氣促(cu)進(jin)了陰(yin)(yin)(yin)、陽(yang)(yang)(yang)極(ji)(ji)反(fan)應(ying)過程,使陰(yin)(yin)(yin)、陽(yang)(yang)(yang)極(ji)(ji)極(ji)(ji)化(hua)(hua)曲(qu)線(xian)(xian)到(dao)達(da)B2和(he)(he)A2,此時(shi)曲(qu)線(xian)(xian)有(you)(you)(you)兩(liang)個(ge)(ge)(ge)(ge)交點(dian),即(ji)出(chu)現(xian)兩(liang)個(ge)(ge)(ge)(ge)自(zi)腐蝕(shi)電(dian)位(wei)(wei)。