1. 動電位極化曲線分析(xi)
圖5.14為不同固溶溫度下2205雙相不銹鋼在0.5mol/L 硫酸溶液中的極化曲線,從圖中可以看出,不同固溶溫度下的試樣極化曲線形狀相似,在陽極區都有一個很寬的鈍化區間,并且鈍化區寬度基本相同,均在-0.2~0.9V之間。這是由于硫酸是一種氧化性酸,雙相不銹鋼中Cr元素含量較高,Cr元素不僅可以降低雙相不銹鋼鈍化的難度,而且可以提高鈍化膜的穩定性,因此,處于0.5mol/L 硫酸溶液環境中在陽極溶解的過程中會發生鈍化。其具體擬合值如表5.5所列。
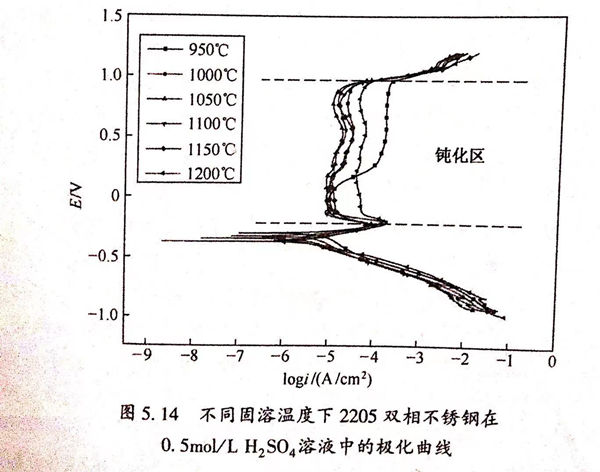
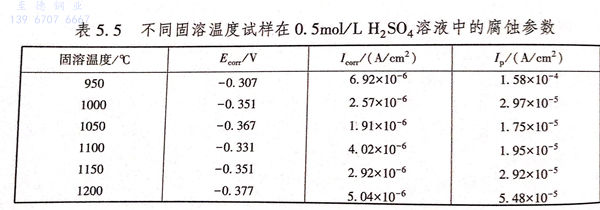
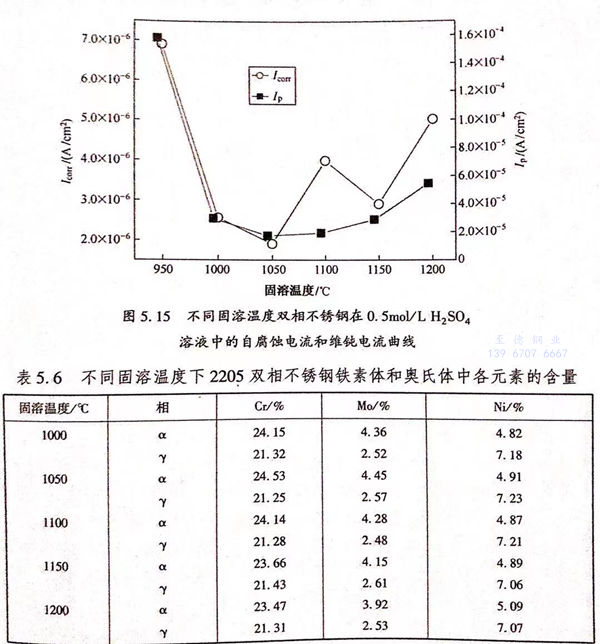
表5.5中Esorr代表自腐蝕電位,Icorr代表自腐蝕電流Ip代表維鈍電流,自腐蝕電位只能代表材料的耐蝕傾向,而自腐蝕電流則可表示材料在溶液中的實際腐蝕速率。由表5.5中數據可知,不同固溶處理溫度下試樣的自腐蝕電位均在-0.4~-0.3V之間,自腐蝕電流大小均為10-6級別,這表明固溶溫度對雙相不銹鋼在硫酸溶液中的耐蝕性能沒有本質的改變,但是也有一些影響。當固溶溫度為950℃時,自腐蝕電流為6.92×10-6(A/c㎡),為所有固溶溫度試樣的最大值;當固溶溫度為1050℃時,自腐蝕電流為1.91×10-6(A/c㎡),為所有固溶溫度試樣的最小值。這表明,當固溶溫度為1050℃時,2205雙相不銹鋼在0.5mol/L 硫酸溶液中耐蝕性能達到最佳;當溫度為950℃時,由于σ相的影響,導致雙相(xiang)不銹鋼耐蝕性能變差。
維鈍電流密度的大小可以反映出材料鈍化膜的穩定性,維鈍電流密度越大說明鈍化膜穩定性越差。因此,當固溶溫度為950℃時,維鈍電流密度為1.58×10-4(A/c㎡),比其他固溶溫度下試樣的維鈍電流密度大了一個數量級,為所有固溶溫度試樣的最大值;當固溶溫度為1050℃時,維鈍電流密度為1.75×10-5(A/c㎡),為所有固溶溫度試樣的最小值。這表明1050℃固溶溫度下,在0.5mol/L 硫酸溶液中材料表面形成的鈍化膜最穩定也最致密;當溫度為950℃時,在0.5mol/L 硫酸溶液中材料表面形成的鈍化膜最不穩定,這是因為σ相的析出導致鐵素體與奧氏體中的Cr元素偏聚其中,導致σ相周圍形成貧Cr區,Cr元素為鈍化膜形成的組要元素,因此,材料表面不能形成很好的鈍化膜,鈍化膜的耐蝕性能下降。
圖5.15為不(bu)(bu)同固溶(rong)溫(wen)(wen)度(du)雙相(xiang)(xiang)不(bu)(bu)銹鋼(gang)(gang)在0.5mol/L 硫酸(suan)溶(rong)液中(zhong)(zhong)自腐(fu)蝕(shi)電(dian)(dian)(dian)流和(he)維(wei)鈍(dun)電(dian)(dian)(dian)流曲(qu)線(xian),從(cong)圖中(zhong)(zhong)可以看出(chu),自腐(fu)蝕(shi)電(dian)(dian)(dian)流和(he)維(wei)鈍(dun)電(dian)(dian)(dian)流具(ju)有(you)相(xiang)(xiang)同的(de)(de)趨勢,隨(sui)(sui)著固溶(rong)溫(wen)(wen)度(du)的(de)(de)增加,2205雙相(xiang)(xiang)不(bu)(bu)銹鋼(gang)(gang)的(de)(de)自腐(fu)蝕(shi)電(dian)(dian)(dian)流和(he)維(wei)鈍(dun)電(dian)(dian)(dian)流均(jun)先(xian)下降后(hou)上升(sheng)。當溫(wen)(wen)度(du)為950℃時(shi),材(cai)(cai)料(liao)的(de)(de)耐(nai)(nai)蝕(shi)性(xing)能(neng)(neng)和(he)鈍(dun)化膜穩(wen)定(ding)(ding)性(xing)均(jun)為最差(cha),主要是由(you)于σ相(xiang)(xiang)的(de)(de)析出(chu)所導致。當固溶(rong)溫(wen)(wen)度(du)達(da)到(dao)(dao)1000℃后(hou),σ相(xiang)(xiang)消(xiao)失,雙相(xiang)(xiang)不(bu)(bu)銹鋼(gang)(gang)中(zhong)(zhong)只存在鐵(tie)素(su)(su)體(ti)(ti)(ti)與奧氏(shi)(shi)體(ti)(ti)(ti)兩(liang)相(xiang)(xiang),消(xiao)除(chu)了第(di)二相(xiang)(xiang)給材(cai)(cai)料(liao)耐(nai)(nai)蝕(shi)性(xing)能(neng)(neng)帶來的(de)(de)負面影響,其耐(nai)(nai)蝕(shi)性(xing)能(neng)(neng)和(he)鈍(dun)化膜穩(wen)定(ding)(ding)性(xing)均(jun)較950℃時(shi)有(you)明顯提高。當溫(wen)(wen)度(du)為1050℃時(shi)耐(nai)(nai)蝕(shi)性(xing)能(neng)(neng)和(he)鈍(dun)化膜穩(wen)定(ding)(ding)性(xing)達(da)到(dao)(dao)最佳(jia)(jia),此時(shi)雙相(xiang)(xiang)不(bu)(bu)銹鋼(gang)(gang)兩(liang)相(xiang)(xiang)比例基本達(da)到(dao)(dao)1:1.表5.6為各固溶(rong)溫(wen)(wen)度(du)下2205雙相(xiang)(xiang)不(bu)(bu)銹鋼(gang)(gang)鐵(tie)素(su)(su)體(ti)(ti)(ti)和(he)奧氏(shi)(shi)體(ti)(ti)(ti)Cr、Mo、Ni的(de)(de)元素(su)(su)含(han)(han)量(liang),由(you)表可知,奧氏(shi)(shi)體(ti)(ti)(ti)中(zhong)(zhong)Cr、Mo元素(su)(su)含(han)(han)量(liang)基本相(xiang)(xiang)同,而(er)鐵(tie)素(su)(su)體(ti)(ti)(ti)中(zhong)(zhong)Cr元素(su)(su)含(han)(han)量(liang)和(he)Mo元素(su)(su)含(han)(han)量(liang)最高,即(ji)此時(shi)各元素(su)(su)在兩(liang)相(xiang)(xiang)中(zhong)(zhong)的(de)(de)分布達(da)到(dao)(dao)最佳(jia)(jia)狀(zhuang)態。隨(sui)(sui)著溫(wen)(wen)度(du)的(de)(de)繼續升(sheng)高,自腐(fu)蝕(shi)電(dian)(dian)(dian)流和(he)維(wei)鈍(dun)電(dian)(dian)(dian)流均(jun)上升(sheng),并與1200℃時(shi)達(da)到(dao)(dao)另外一個(ge)峰值(zhi)。由(you)于隨(sui)(sui)著固溶(rong)溫(wen)(wen)度(du)的(de)(de)升(sheng)高,鐵(tie)素(su)(su)體(ti)(ti)(ti)與奧氏(shi)(shi)體(ti)(ti)(ti)兩(liang)相(xiang)(xiang)比例逐漸(jian)偏(pian)離1:1,而(er)從(cong)表5.6中(zhong)(zhong)可以看出(chu)此時(shi)鐵(tie)素(su)(su)體(ti)(ti)(ti)含(han)(han)量(liang)不(bu)(bu)斷增加,奧氏(shi)(shi)體(ti)(ti)(ti)含(han)(han)量(liang)逐漸(jian)降低,鐵(tie)素(su)(su)體(ti)(ti)(ti)中(zhong)(zhong)Cr和(he)Mo元素(su)(su)含(han)(han)量(liang)降低,各元素(su)(su)在兩(liang)相(xiang)(xiang)中(zhong)(zhong)的(de)(de)分布偏(pian)離最佳(jia)(jia)狀(zhuang)態。因此,其耐(nai)(nai)蝕(shi)性(xing)和(he)鈍(dun)化膜穩(wen)定(ding)(ding)性(xing)均(jun)變差(cha)。
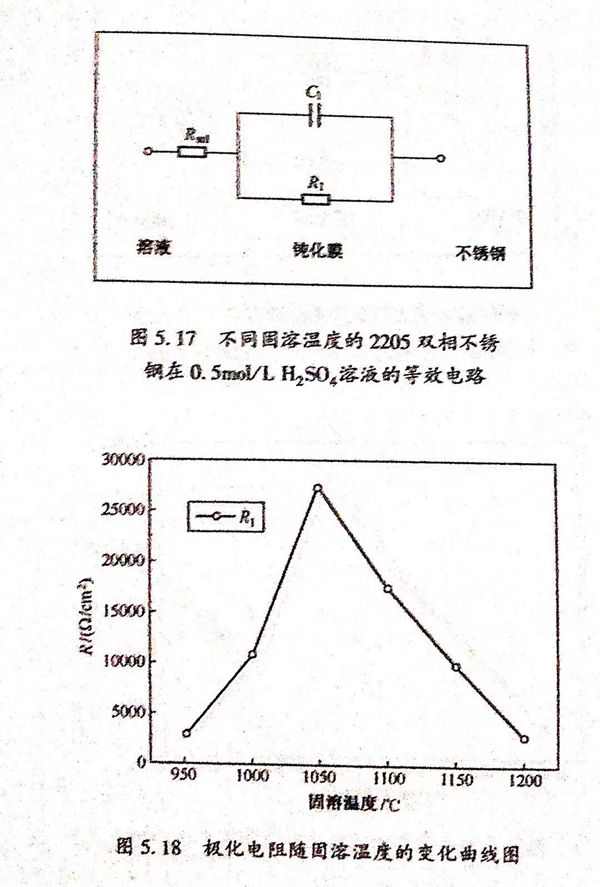
2. 交流(liu)阻抗測試分析
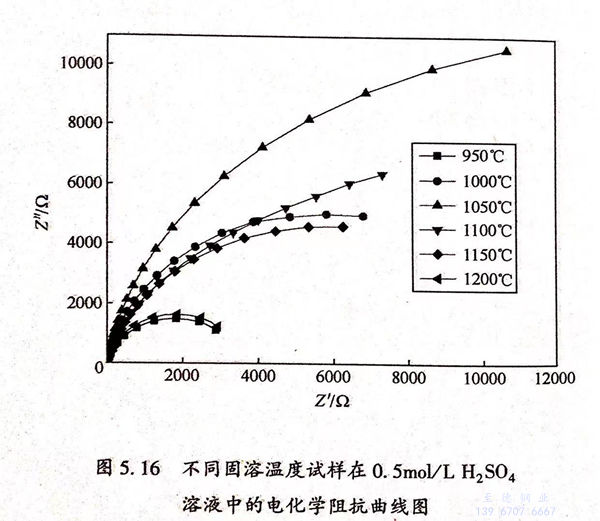
圖5.16為不同固(gu)(gu)溶溫(wen)度試樣(yang)在0.5mol/L 硫酸(suan)溶液(ye)中的(de)(de)(de)(de)電化學阻抗譜Nyquist 曲線圖。從圖5.16中可知(zhi),所有(you)固(gu)(gu)溶溫(wen)度下試樣(yang)的(de)(de)(de)(de)Nyquist 曲線均由~個較(jiao)(jiao)大(da)的(de)(de)(de)(de)半(ban)(ban)圓弧構(gou)成(cheng)。比(bi)較(jiao)(jiao)半(ban)(ban)圓弧的(de)(de)(de)(de)直(zhi)(zhi)徑(jing)可知(zhi):1050℃>1000℃>1100℃>1150℃>1200℃>950℃.Nyquist曲線半(ban)(ban)圓弧的(de)(de)(de)(de)直(zhi)(zhi)徑(jing)代(dai)表了材(cai)料(liao)耐(nai)(nai)蝕(shi)性能,直(zhi)(zhi)徑(jing)越大(da)說明材(cai)料(liao)耐(nai)(nai)蝕(shi)性能越好(hao)。因此,材(cai)料(liao)在1050℃時耐(nai)(nai)蝕(shi)性能最好(hao),950℃時耐(nai)(nai)蝕(shi)性能最差,這與極(ji)化曲線的(de)(de)(de)(de)結果相一(yi)致。
不(bu)同固溶(rong)(rong)溫(wen)度下2205雙(shuang)相不(bu)銹(xiu)鋼(gang)阻(zu)抗(kang)等效電(dian)(dian)路(lu)和擬合數據(ju)如圖5.17和表(biao)5.7所示。表(biao)5.7中(zhong)Rsol為(wei)(wei)溶(rong)(rong)液(ye)(ye)電(dian)(dian)阻(zu),Cl為(wei)(wei)雙(shuang)電(dian)(dian)層(ceng)電(dian)(dian)容,Rl為(wei)(wei)極化(hua)電(dian)(dian)阻(zu)。溶(rong)(rong)液(ye)(ye)電(dian)(dian)阻(zu)在2~6Ω/c㎡內波動,相比較極化(hua)電(dian)(dian)阻(zu)可以忽(hu)略不(bu)計,說明溶(rong)(rong)液(ye)(ye)本(ben)身的(de)影響很小(xiao)。極化(hua)電(dian)(dian)阻(zu)R1隨固溶(rong)(rong)溫(wen)度的(de)變化(hua)曲線如圖5.18所示。從圖5.18中(zhong)可以看出,R1在1050℃達到最(zui)大(da)值27290Ω/c㎡,在950℃達到最(zui)小(xiao)值2579Ω/c㎡,并(bing)且隨著固溶(rong)(rong)溫(wen)度的(de)升高先(xian)增(zeng)大(da)后減(jian)小(xiao)。表(biao)明當固溶(rong)(rong)溫(wen)度達到1050℃時,雙(shuang)相不(bu)銹(xiu)鋼(gang)的(de)鈍化(hua)膜穩定性和致密程度最(zui)佳,與溶(rong)(rong)液(ye)(ye)進行反應的(de)速度最(zui)小(xiao),反應難度最(zui)大(da)。這與極化(hua)曲線得到的(de)結果相致。